Consortium
The project involves:
- Two University Hospitals:
- Hospices Civils de Lyon (HCL)
- CHU de Saint-Étienne (CHUSE)
- Five academic partners:
- A private company:
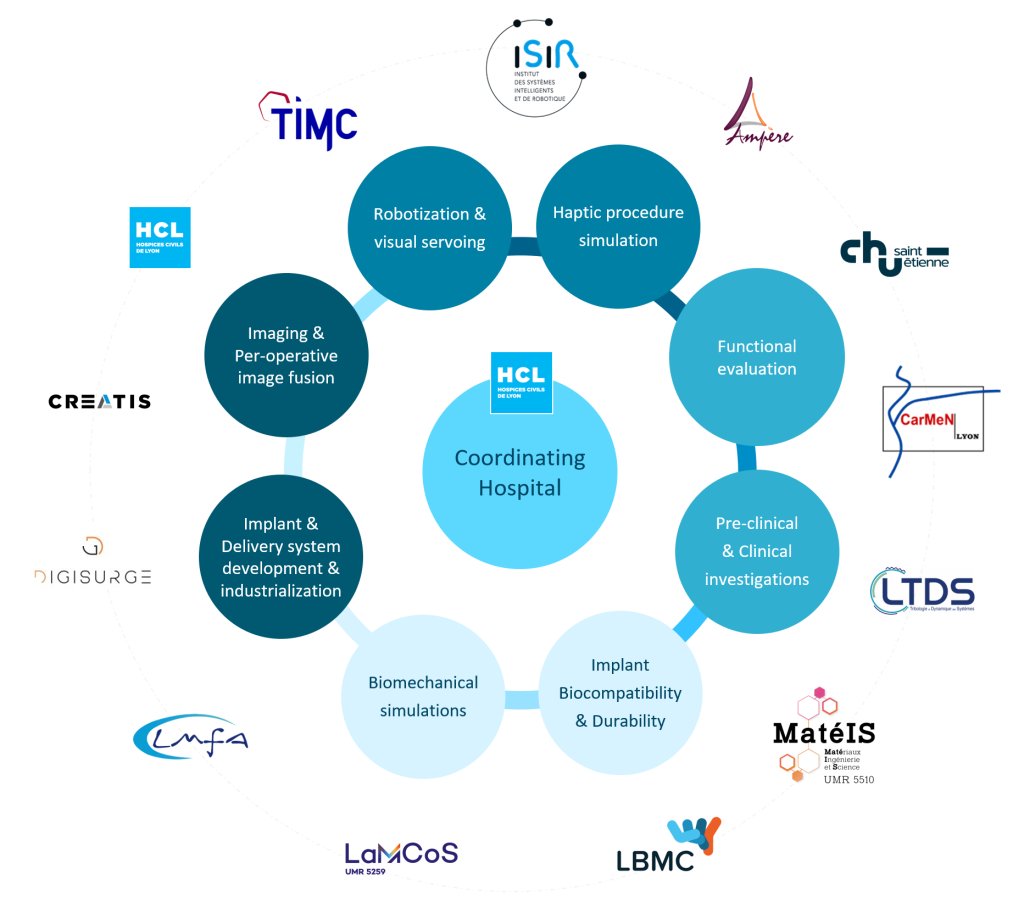
Consortium governance
The governance of ICELAND is composed of two entities:
- A Steering Committee composed by the project coordinator, the project manager and the stakeholders of the project.
- An independent Scientific Advisory Board in charge of scientific advising.
